Monucell- Heart failure in cell therapy
Cell therapy is the safest and most secure treatment
Heart failure is a condition in which the heart is unable to pump enough blood to different tissues in the body. In simpler terms, heart failure means a drastic reduction in the function of the heart, meaning that the contraction or expansion of the heart is reduced and it cannot pump enough blood. A heart attack is the most common cause of heart failure that destroys both some heart muscle cells and the ability of these cells to contract. Therefore, the contraction power of the heart decreases. In the early stages of the disease, the patient may not exhibit any visible symptoms. However, it may gradually develop as the patient shows sign of premature fatigue, shortness of breath, decreased energy, shortness of breath during activity, and in more severe cases when the patient is at rest, palpitations, and irregular heartbeats.
Some treatments for heart failure, include medications, surgery, and in more severe cases, a heart transplant. Prolonged treatment, high costs, and risks during operation are some of the reasons that researchers are looking for new ways to treat patients with heart failure.
More information for physicians
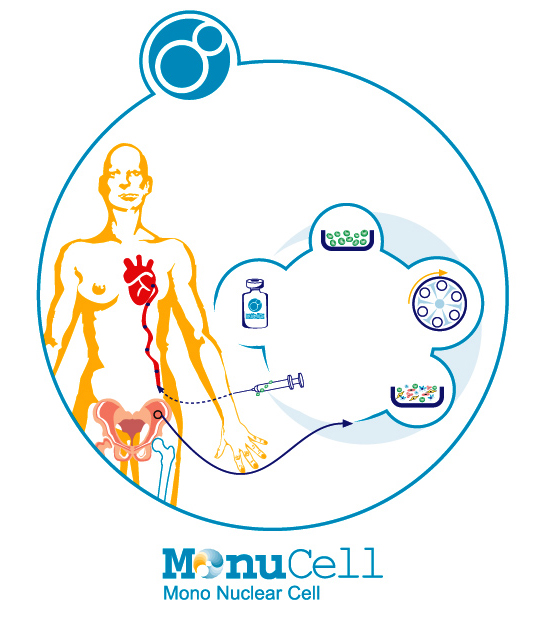
Studies have shown that transplantation of stem cells into the heart muscle of patients whose had heart attack can improve heart function and quality of life. In collaboration with other scientific centres, cell therapy in cardiac patients at Royan Research Institute began in 2007 on patients with acute stroke and heart failure. In this method, a tissue sample is first removed from the patient’s bone marrow and transferred to Celltech Pharmed-Stem Cell factory. During 3 to 4 hours, the patient’s stem cells are separated and then transplanted through the coronary artery or directly to the patient’s heart muscle.
Celltech Pharmed-Stem Cell company calls these processed cells Minucell, which are the product of the bone marrow cells of patients. Compatibility of this cellular product with the body, short recovery period, no complication during treatment and no need for injections of the cells in most cases are among the reasons that specialists have considered this approach extremely reliable for the treatment of patients with heart failure.
FAQ
What types of stem cells are used to treat heart disease?
All three types of stem cells, including embryonic stem cells (ESCs), somatic stem cells and induced stem cells are used to repair heart lesions. But only some types of second-hand cells, such as somatic stem cells, are licensed for use in humans and clinical practice.
What types of somatic stem cells are used for heart disease?
In clinical trials currently being conducted at many research centres around the world, six significant types of stem cells have been proposed to repair cardiovascular tissue.
- The first type is Skeletal Myoblasts, which are derived from skeletal muscle. The first stem cells that were used in the year 2000 in heart disease. However, due to several mild arrhythmias, they are less commonly used today.
- The second type, mononuclear cells, are isolated from bone marrow and are the most widely used cells since 2001 due to their secure processing and without culturing and side effects. These cells are made up of heterogeneous populations, including vascular progenitor cells and hematopoietic stem cells. These cells are produced and under the name of Minucellby Celltech Pharmed-Stem Cell company.
- The third type is mesenchymal stem cells, which are derived from the bone marrow and because of their high ability to divide and differentiate are very well considered.
- The fourth type is CD34 and CD133 cells that due to their special proprietary features, like the high ability of angiogenesis will be separated from unicellular cell population derived from bone marrow.
- The fifth type is cells that have been derived from the body’s adipose tissue that their advantage is the ease of access to resources and their abundance.
- The sixth type is stem cells inhabiting the human heart, which are the newest type of stem cells used in heart disease. In July 2012, the US Food and Drug Administration confirmed the first clinical study. In this approach, these cells are used to repair the damage caused by heart attacks.
What is the primary source of stem cells, and how are they obtained?
Bone marrow: In this procedure, to take a sample from the hip bone, anesthetic is first injected. Then, bone marrow samples are taken using special needles for aspiration and sent to the laboratory for further preparation.
Adipose tissue: In this procedure, some of the excess body fat in the abdomen, thighs or sides is aspirated with a syringe.
Heart tissue: In this method, without opening the chest, a few tiny samples of the healthy area of the patient’s heart tissue are taken in a similar way to angiography.
How are processed stem cells injected into a patient?
According to studies that have been conducted among a variety of injection methods, two methods, intramuscular injection and intravascular injection have the most effective outcome. However, intramuscular injections can only be given to patients who are candidates for open-heart surgery; otherwise, the cells are injected into the heart vessels and through angiography.
How long does it take for stem cells to show a healing effect?
According to studies, the beginning of the healing process of these cells is almost three months after the injection, but this effect can gradually increase for up to one year.
Based on what criteria do stem cells show an improvement in cardiac function?
This improvement can be seen in the following three general categories.
Prevention of adverse events such as death, recurrent heart attack, frequent hospitalization due to disease complications and heart transplantation.
Improving physical activity, quality of life, greater effectiveness of medications and patient satisfaction after treatment.
Improving paraclinical criteria, including cardiac imaging on echocardiography, scanning, MRI, as well as special blood tests such as (Pro-BNP).
Can stem cells be used to treat all cardiovascular diseases?
No. Currently, these cells are most commonly used for ischemic heart disease, including heart attack and heart failure. However, the use of stem cells in acute myocardial infarction, adult ischemic heart failure, adult dilated heart failure, pediatric dilated heart failure, mitral valve coronary failure, lower limb ischemia, and lymphatic vessel swelling (lymphedema) is being investigated.
Is the use of this method provided in all medical centres?
Currently, this method is used only in Shahid Rajaei Cardiovascular Hospital, Cardiac Center, Baqiyatallah Hospitalو Lavasani, Masih Daneshvari, Sasan and Bahman in Tehran province, as well as Imam Reza hospital, Montaseriyeh Hospital, Razavi and Javadol A’emmeh Hospital in Mashhad. The procedure can be performed in other hospitals if the necessary preparations are made.
Does this treatment have any particular side effects for the patient?
Although no method is considered 100% safe, no specific side effects have been reported so far.