Mesestrocell- Cell therapy for osteoarthritis
Cell therapy is the safest and most secure treatment
Osteoarthritis is the most common inflammatory disease in the joints, affecting more than half of the population over the age of 60. In this disease, the cartilage of the joint is damaged and gradually disappears. As a result, the wear of the bones can cause pain, swelling, and loss of ability. The most common cause of osteoarthritis in the knee is aging. Many factors such as overweight, heredity and trauma can also increase the risk of developing osteoarthritis at an early age. Any joint can be affected by osteoarthritis, but large joints, such as the knee, thigh, and spine that support body weight are more likely to be affected. When joint pain starts, it makes exercising and physical activity difficult. Conventional treatments include medication, injections of hyaluronic acid gel, and joint replacement surgery that may have several side effects. If the joint is severely damaged, your doctor may recommend a joint replacement. One of the most critical complications of knee replacement surgery which is regarded as an invasive procedure is a lengthy recovery period and severe pain. Today, cell therapy has revolutionized the process of treating patients.
More information for physicians
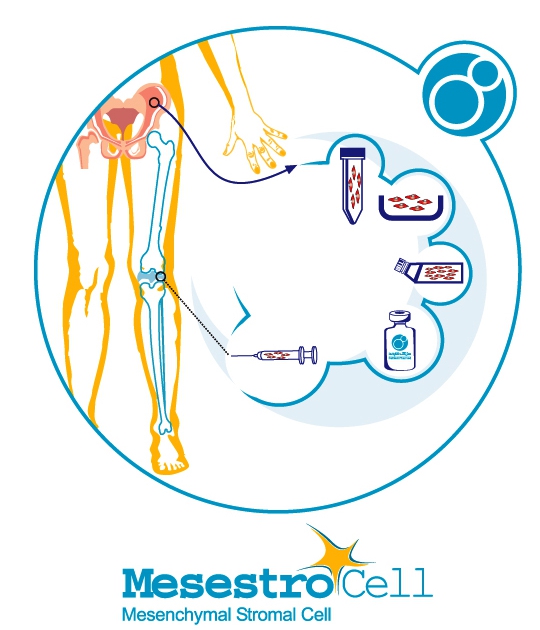
The knowledge advancement of stem cell and reconstructive medicine has opened a new way into modern medicine. Mesenchymal stem cells can help patients who have developed osteoarthritis by two distinctive feature; the ability to differentiate into cartilage cells and to modulate immune responses. In this procedure, stem cells will be extracted from the bone marrow of the patients and then will be cultured in an entirely sterile laboratory. The final product which will be ready for the injection into the knee is called Mesestrocell, which is manufactured by Celltech Pharmed-Stem Cell company. After the injection of Mesestrocell, the steps to recovery begins gradually after six months which will be observable from monitoring the recovery progression through MRI imaging and the patient’s motor movement. The most crucial advantage of cell therapy is the health and reliability of the treatment because stem cells are taken from the patient’s own body and cultured with unique control and precision.
Importantly, the Mesestrocell product contains live mesenchymal stromal which have been cultured from the patient’s bone marrow tissue. Moreover, short-term recovery period, no need to repeat the injection and to have little pain during the treatment period are some of the advantages of this treatment method.
Since many professional athletes are at risk of injury during their training and may stay away from exercising for a long time, the short recovery period is strategically essential. So far, many athletes have undergone cell transplantation, including Cristiano Ronaldo, Rafael Nadal and Chris Johnson.